The Dead Sea puts pressure on your ears…
I had the privilege of being accompanied by Hamse and Samir, two employees from the Jordan Children’s Museum. The journey began in the capital of Jordan, Amman, and took barely an hour. The experiments themselves however began before departure, in my hotel room on the sixth floor, approximately 970 metres above sea level. I wanted in particular to measure how big the difference in air pressure is between Amman (more specifically the hotel room) and the Dead Sea (on the beach). At any rate the height difference of around 1,400m should be clearly noticeable. In the same way that water pushes harder on your ears deep underwater compared to on the surface, a bigger air column likewise exerts more pressure.
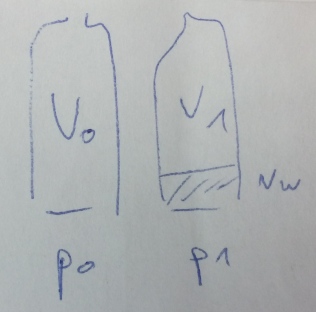
Unfortunately in Amman I couldn’t get hold of a barometer. I really wanted to measure the difference in air pressure, and in the end I was encouraged through the internet chat of the British Interactive Group, an organisation of science communicators, who I had asked for ideas for experiments at the Dead Sea. The solution: a completely normal plastic drinking bottle. In my hotel room I emptied the bottle and sealed the cap on tightly, and simply took it with me to the Dead Sea. The increase in air pressure subsequently resulted in the bottle being squashed a decent amount during the journey.